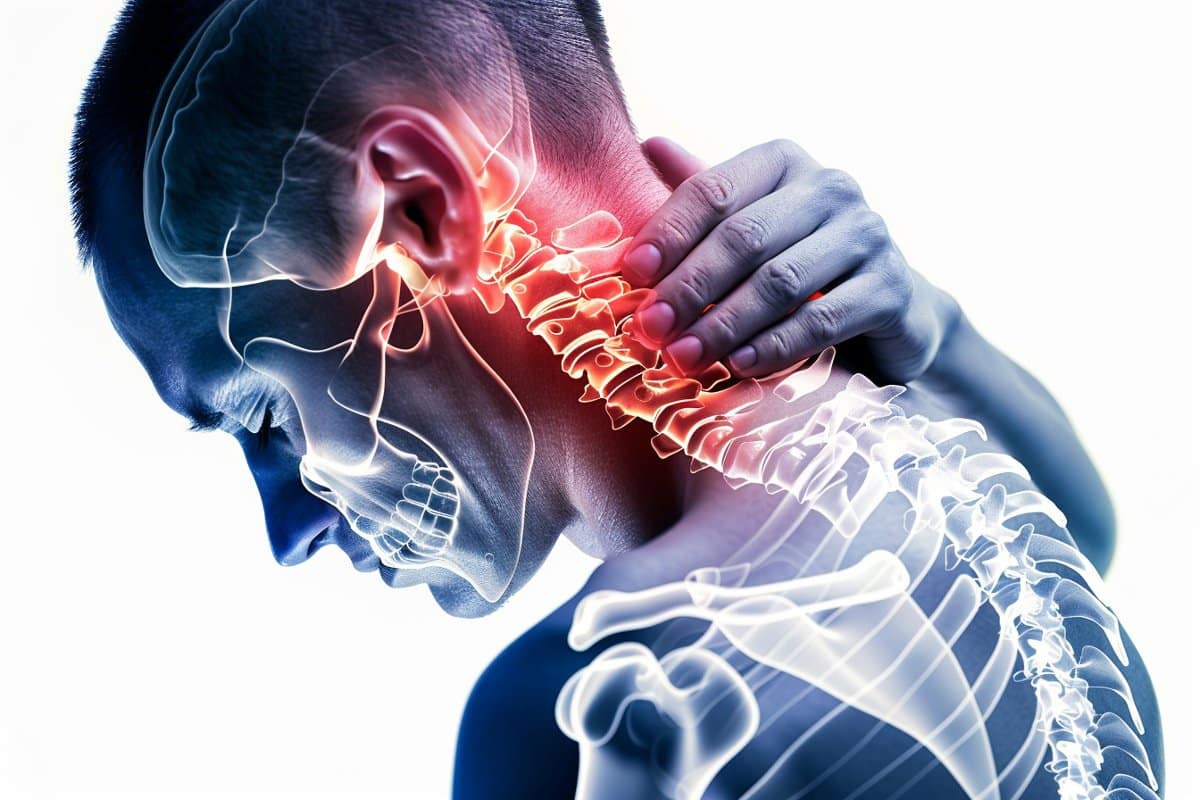
The sensation of pain is intricate and multidimensional, and it differs greatly from person to person. A better understanding of the hereditary basis of pain sensitivity and tolerance may result in more individualized and efficient pain treatment techniques.
The Origins of Pain in Biology
Pain is a necessary biological function that alerts the body to impending or current damage. Which includes intricate interactions between the brain, spinal cord, and sensory neurons. Specialized sensory receptors called nociceptors recognize harmful stimuli and send signals to the spinal cord, brain, and peripheral nerves, where the brain interprets the information as pain.
A number of important neurotransmitters and molecular pathways have a role in controlling pain. These comprise, among other systems, the opioid, serotonergic, and catecholaminergic systems. Different peoples experiences and approaches to managing pain. Greatly influenced by the genes that encode the proteins involved in these processes.
Genetic Distinctions and Sensitivity to Pain
A multitude of genetic variations linked to pain. These variations have the potential to impact pain perception via multiple pathways.
Polymorphisms in Pain-Related Genes: Disparities in pain sensitivity can result from variations in the genes that encode neurotransmitters, pain receptors, and enzymes involved in pain signaling pathways. Pain insensitivity and increased pain sensitivity in certain people.
Opioid Receptor Genes: An essential component of the body’s natural pain management system is the OPRM1 gene, which codes for the mu-opioid receptor.
Catechol-O-Methyltransferase (COMT) Gene:
Dopamine, adrenaline, and norepinephrine are among the catecholamines. Broken down by the COMT enzyme. The COMT gene’s Val158Met polymorphism modifies the activity of the enzyme, which impacts pain perception and vulnerability to chronic pain syndromes.
Voltage-Gated Calcium Channels:
That encode voltage-gated calcium channels, such as CACNA1A and CACNA1B. Which can contribute to diseases like familial hemiplegic migraine.
Pain Sensitivity and Tolerance Heritability
Twin research has shed light on the heredity of pain tolerance and sensitivity. Indicating that a sizable amount of the variation in pain.
Research on artificial pain models, for example, has demonstrated that identical twins, who have 100% of the same genes, display more similar pain responses than fraternal twins, who have 50% of the same genes. There is a genetic component to pain of all kinds, including mechanical, chemical, and thermal pain.
Pain-Related Conditions and Genetic Predisposition
Not only do some genetic variations affect an individual’s sensitivity to pain in general, but they also make them more likely to develop certain pain-related diseases.
Migraine: Genetic research has linked a number of loci, including polymorphisms in the TRPM8 gene, which codes for a receptor implicated in pain and cold perception, to migraine risk. Furthermore, mutations in the CACNA1A, ATP1A2, and SCN1A genes have been connected to familial hemiplegic migraine, an uncommon kind of migraine.
Fibromyalgia: Weariness, soreness, and diffuse musculoskeletal pain are the hallmarks of this chronic pain condition. Studies indicate a hereditary inclination towards fibromyalgia, citing polymorphisms in genes associated with serotonin and dopamine signaling pathways, like DRD4 and SLC6A4.
Pain and Epigenetics
The regulation of gene expression without affecting the underlying DNA sequence is known as epigenetic processes, and it is a key component of both pain sensitivity and tolerance. Stress, nutrition, and exposure to the environment can all alter one’s epigenetic state, which in turn affects how one feels pain.
Consequences for Pain Management
Pain management and therapy will be significantly impacted by our growing understanding of the genetic underpinnings of pain sensitivity and tolerance. Customized pain management, which adjusts dosage according to a patient’s genetic profile, has the potential to enhance pain alleviation and minimize side effects.
Pharmacogenomics:
Medical professionals can choose the best analgesics and dosages for each patient by finding genetic variations that impact drug metabolism and reaction. For instance, since this enzyme metabolizes a large number of opioids, genetic testing for CYP2D6 polymorphisms can help guide the prescribing of opioids.
Gene Therapy:
In an effort to reduce pain, experimental methods like gene therapy try to fix genetic flaws or alter gene expression. These treatments have the potential to treat hereditary pain disorders, even though they are still in the early stages.
Prospective Courses
Research into pain genetics is constantly changing as new genetic variants are found and their functional implications are comprehended. Our understanding of the genetic architecture of pain is growing thanks to next-generation sequencing methods and genome-wide association studies (GWAS).
In order to create complete models of pain, future research will probably concentrate on combining genetic, epigenetic, and environmental data. Improved, tailored treatment plans and more precise assessments of pain susceptibility can result from this integrative approach.
In summary
The vast range of pain experiences experienced by individuals. Attributed to variations in genes related to inflammation, neurotransmission, and pain signaling pathways. Comprehending these genetic variables can result in tailored pain management strategies. Enhancing the results for patients suffering from both acute and persistent pain ailments. The application of genetic insights to clinical practice could revolutionize pain treatment and improve the lives of individuals with pain.